Flipping a molecular switch
HIRI and University of Cambridge shed light onto an essential protein-assisted genetic switch in Cardioviruses / Study published in "Nature Communications"
Würzburg, Germany, December 14, 2021 – Researchers from the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg and the University of Cambridge have revealed the molecular details of a genetic switch through their work on the 2A protein of cardioviruses. Using a new RNA-binding fold, the 2A protein activates a process called ribosomal frameshifting, which allows the virus to replicate more efficiently. This event represents an essential switch in the life cycle of the virus as well as presenting a viral Achilles' heel that could be targeted by future RNA-based therapies. The new findings have just been published in the journal Nature Communications.
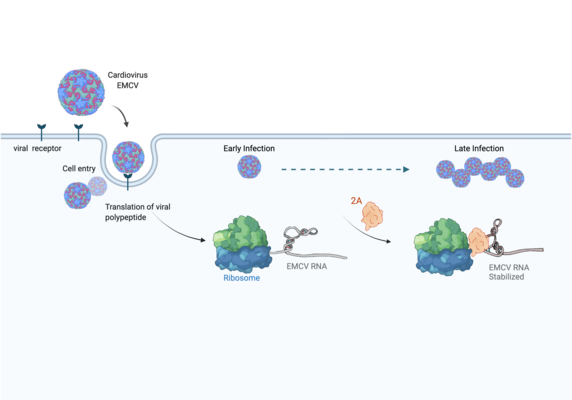
Infections with the encephalomyocarditis virus (EMCV) affect a variety of mammals such as pigs and chimpanzees. However, humans can also become infected, resulting in fever and inflammatory disease. Like other cardioviruses – or like the highly infectious coronavirus SARS-CoV-2 – EMCV is an RNA virus: its genetic material consists of ribonucleic acid (RNA). Since viruses do not have their own metabolism, in order to reproduce they must infect a host cell and take control over its protein synthesis factories: ribosomes.
A forced error: protein-modulated frameshifting
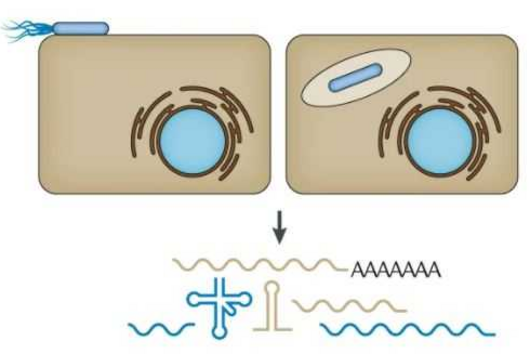
The manipulation of the host cell’s protein factory is achieved by ribosomal frameshifting: When reading and translating the genetic information in the messenger RNA, a proportion of ribosomes are forced into a new reading frame. This changes the way the entire sequence is decoded and allows more than one protein to be synthesized from a single messenger RNA.
"By hijacking the host's translation engine and corrupting the system to produce their own proteins, viruses have proven to be fearsome cellular invaders," says Neva Caliskan, research group leader at the Würzburg Helmholtz Institute for RNA-based Infection Research (HIRI) and co-corresponding author of the study.
"In our current work, we focused on investigating how this process is regulated in EMCV replication. The multifunctional 2A protein has a key role in this process," says Ian Brierley, group leader at the University of Cambridge (Department of Pathology) and co-corresponding author of the study.
Scientists at the University of Cambridge have solved the crystal structure of 2A and revealed how it interacts with ribosomes by cryo-EM. Chris Hill, one of the leading authors of the study and now a new group leader at the University of York, explains “the fold of this protein doesn’t resemble any other known RNA-binding proteins, so we call it the ‘beta-shell’. With these structural blueprints, we can better understand years of previous work on how this multi-functional protein can interact with its targets.”
Researchers were also able to show how this folding contributes to the interactions of 2A with the viral RNA. "The docking of the 2A protein to the RNA stabilizes its structure and forces a frameshift," explains Lukáš Pekárek, who is one of the first authors of the scientific study. "We were able to observe this effect in detail, at high resolution through our optical tweezers," says the HIRI PhD student in the Caliskan lab.
"At the beginning of an EMCV infection, the 2A protein is not yet present; instead, other proteins that the virus needs to replicate its genome are active," says HIRI PhD student Anuja Kibe, who also contributed to the study. As the infection progresses, she says, 2A accumulates in the cells and stimulates the frameshift. With that, the pathogen can finely adjust the amounts of different proteins required to assemble more viruses.
Further research can address this viral Achilles' heel to develop targeted RNA technologies and therapeutics and bring them into medical use.
About Cardioviruses
Cardioviruses are among the smallest viruses in the world. They have a diameter of about 22 to 30 nanometres, approximately a thousand times smaller than the diameter of a hair. This is far too small to visualize viruses of this size under a light microscope. RNAs, or ribonucleic acids, which make up the genome of these viruses, are even smaller. Scientists at HIRI therefore work with single molecule optical tweezers, among other cutting-edge technologies. These make it possible to examine molecular structures and RNA functions in atomic resolution.
About the HIRI
The Helmholtz Institute for RNA-based Infection Research (HIRI) is the first institution worldwide to combine ribonucleic acid (RNA) research with infection biology. Based on novel findings from its strong basic research program, the institute's long-term goal is to develop innovative therapeutic approaches to better diagnose and treat human infections.
HIRI is a location of the Helmholtz Centre for Infection Research (HZI) in Braunschweig in cooperation with the Julius-Maximilians-Universität Würzburg (JMU) and is located on the Würzburg Medical Campus.
Publication
Hill C H, Pekárek L, Napthine S, Kibe A, Firth A E, Graham S C, Caliskan N, Brierley I. Structural and molecular basis for Cardiovirus 2A protein as a viral gene expression switch. Nature Communications, Dec. 9, 2021. DOI: 10.1038/s41467-021-27400-7.
The study was supported by funding from the Wellcome Trust, the Helmholtz Association and the European Research Council, among others.

Press contact